Understanding lubricant pour point depressants
Understanding lubricant pour point depressants
As part of an ongoing exploration of additives with WearCheck’s condition monitoring specialists, this article focuses on how pour point depressants (PPDs) impact oil rheology, or flow behaviour.
PPDs prevent oil congelation at low temperatures due to wax crystallisation. “They do this by modifying the interface between the crystallised wax and the oil. Examples of PPDs include polymethacrylates (PMAs), alkylated wax naphthalene, and alkylated wax phenol,” explains Steven Lumley, technical manager for WearCheck.
“Medically, ‘depressant’ is synonymous with prescription drugs that soothe frayed nerves by lowering neurotransmission. In chemistry, however, a depressant is any agent capable of diminishing a specific property of a substance. A pour point depressant is therefore an additive that depresses the pour point of a lubricant,” she continues.
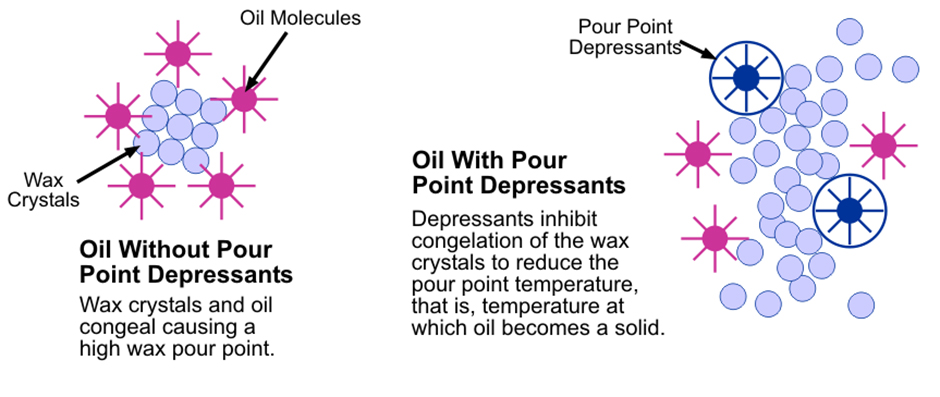
“An oil’s pour point is the lowest temperature at which it remains fluid. Wax crystals that form in paraffinic mineral oils crystallise (solidify) at low temperatures. These solid crystals form a lattice network that inhibits the remaining liquid oil from flowing,” Lumley expands.
In temperatures below freezing, all paraffinic oils form wax crystals, which can hamper the oil’s fluidity (ability to flow). PPDs don’t stop crystal formation, but they do prevent wax crystals from agglomerating by reducing both the size of the crystals in the oil and their interaction with each other, allowing the oil’s continued fluidity at low temperatures.
“PPDs are polymeric molecules added to mineral oil-based lubricants that are exposed to low temperatures. They prevent a viscosity increase so that the oil will not flow too much and starve the system of necessary lubricant, especially on startup,” says Lumley.
In fact, the viscosity can even increase, such that the oil “gels”, or becomes semi-solid. Critical applications include engine, transmission, gear, and hydraulic lubricants. Many of these lubricants have predominantly paraffinic base stocks, which contain significant wax concentrations that cause pumpability problems at low temperatures. Waxy components are also added to a formulation as detergent components, or in some viscosity index improver packages.
At low temperatures below the cloud point – the temperature at which an oil’s dissolved solids like paraffin wax begin to form and separate from the oil – these waxy molecules cause a rapid increase in the oil’s viscosity, and the waxy molecules begin to crystallise. The oil clouds and, ultimately, these wax crystals can precipitate from the lubricant. PPDs slowly increase the oil’s viscosity with decreasing temperature, as above the cloud point, but prevent the rapid increase in viscosity associated with waxy crystal formation.
As noted by Lumley, PPDs are polymeric materials. These are made from many different polymer chemistries such as acrylates, styrenes, alpha olefins, vinyl acetates, and others. “These chemistries, when polymerised, form a linear backbone that can have side chains with varying long (waxy) and short (non-waxy) hydrocarbons. Imagine the polymers similar to a hair comb, but with long and short teeth. This structure inhibits the formation of wax crystals by virtue of the long-chain portions of the PPD partially co-crystallising with the wax, and the short chains aiding in solubilising the lubricant’s complex,” she elaborates.
“The polymethylmethacrylates (PMAs) are a dominant chemistry used for PPDs. They have an inherent oxidative and thermal stability, making them ideal for engine oils. This meets automotive OEM requirements for low temperature pumpability of used engine oils,” she says. “In addition to concern about wax crystal formation, there are the effects of oxidation on the oil-forming polar molecular species, that can also form gels and raise the used oil’s viscosity. Thus, PPDs are hardly depressing but are one more tool formulator that enhances lubricants’ performance in engines, transmissions, gears, and hydraulics.”
Published by
Focus on Transport
focusmagsa




